Title:Precision Surface Chemistry to Optimize Near Surface Quantum Bits in Diamond and Emergent Quantum Workforce Development Sensing, computation and communication based on quantum physics are emergent fields and atomic defects hosted in diamond are a model system to explore fundamental phenomenon in these areas at room temperature with visible light. National security and warfighter superiority will likely hinge on the developments in quantum-based technology in the coming decades. There are a number of challenges with near-surface nitrogen vacancy (NV) centers in bulk and nanoscale diamond that produce reduced electron-spin coherence times and inhibit the sensing capabilities of the NV center. This proposal addresses these obstacles with unique chemistry to modify the diamond surface’s electronic structure and uses fundamental methods to interrogate the spin dynamics of NV centers. Two aims of the project include: 1) fundamental surface science, robust chemical analysis and NV photophysics with 2) the training of an ultra-diverse student body for the emergent quantum work force. The Wolcott lab at San José State University (SJSU) will leverage their expertise in diamond surface chemistry while expanding research capacity through a long-standing collaboration with Stanford Synchrotron Radiation Lightsource (SSRL) scientists and a new collaboration with the Meriles group at City College of New York.
In the quest to activate the surface of nanoscale diamond, Wolcott lab researchers collaborated with The Molecular Foundry at Lawrence Berkeley National Lab. The Foundry, as its known, is one of 5 nanoscience centers operated by The Department of Energy. In collaboration with staff scientist Dr. Virginia Altoe, the team confirmed that electrophilic boron molecules can readily react with diamond surface groups and create a thin sheet. The thin sheet of boron could be used for cancer therapy applications and in quantum sensing with defects inside of the diamond!
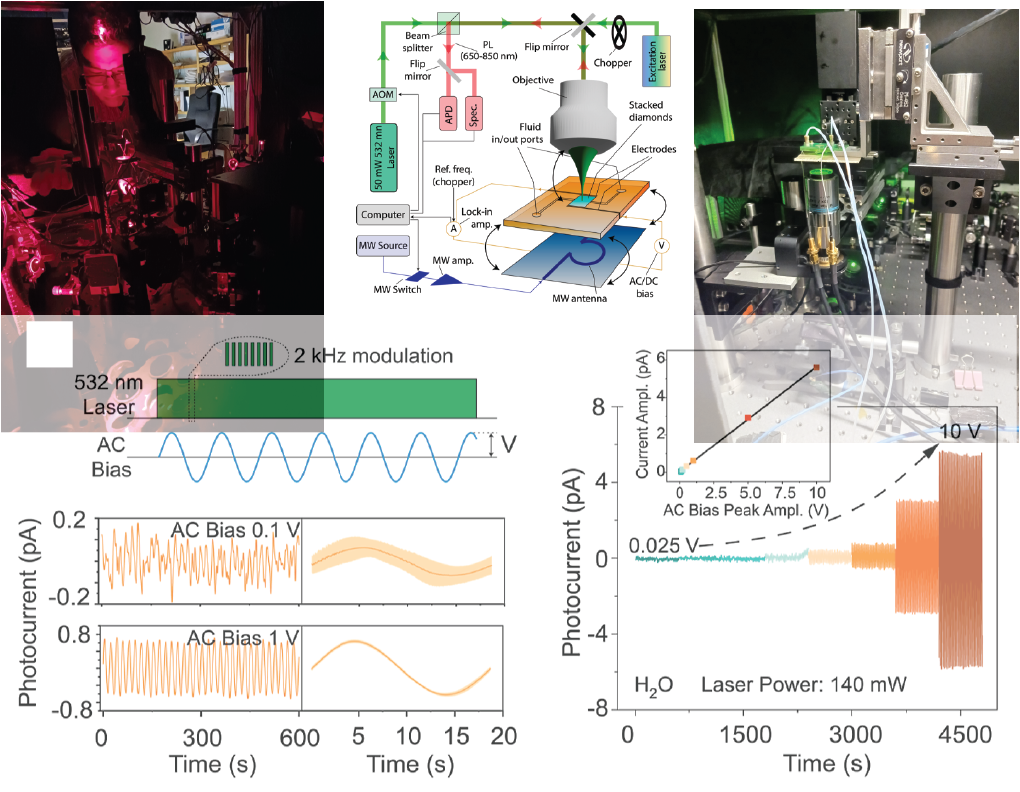
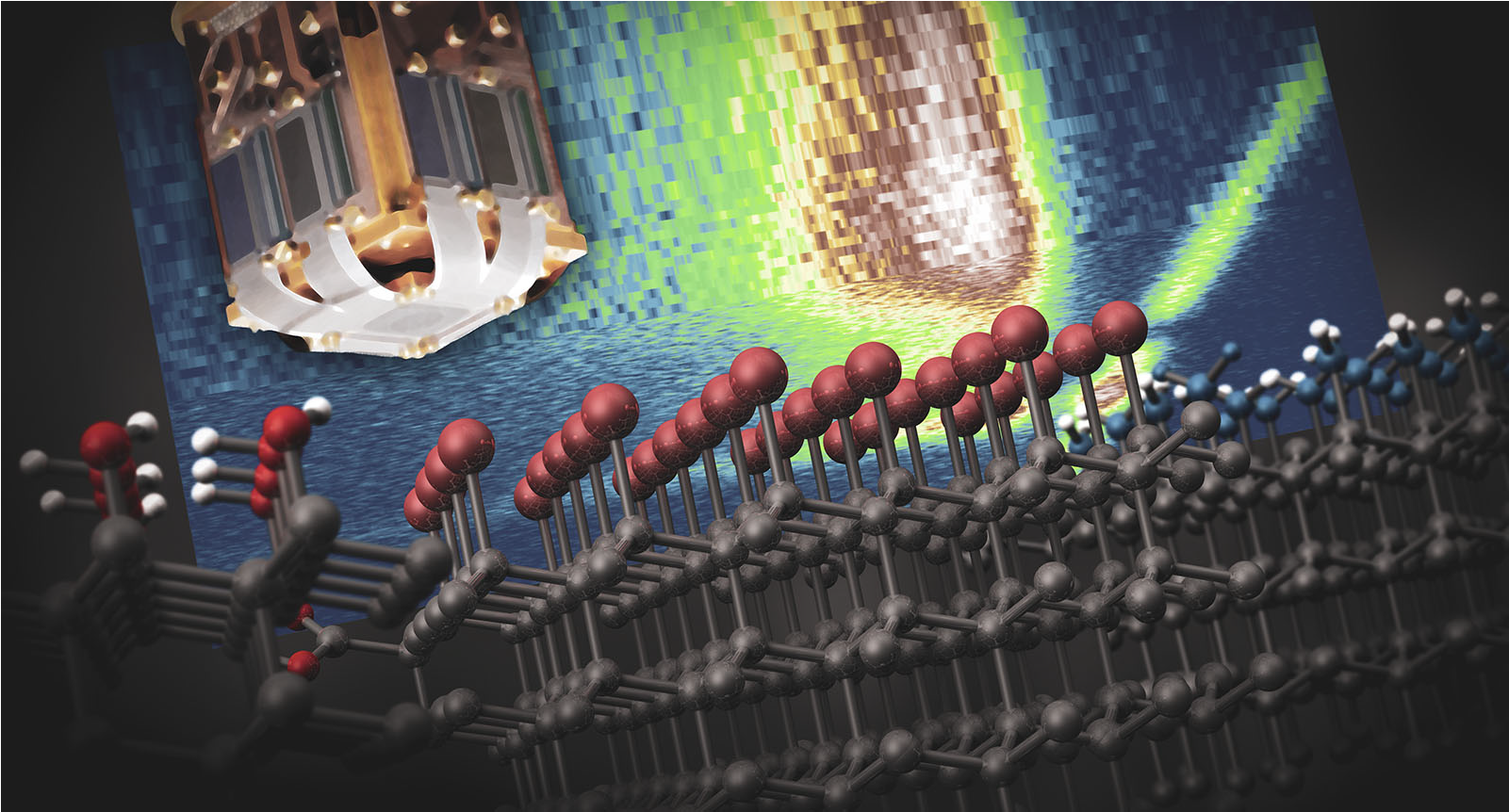
Thanks to its low or negative surface electron affinity and chemical inertness, diamond is attracting broad attention as a source material of solvated electrons produced by optical excitation of the solid−liquid interface. Unfortunately, its wide bandgap typically imposes the use of wavelengths in the ultraviolet range, hence complicating practical applications. Here, we probe the photocurrent response of water surrounded by single- crystal diamond surfaces engineered to host shallow nitrogen- vacancy (NV) centers. We observe clear signatures of diamond- induced photocurrent generation throughout the visible range and for wavelengths reaching up to 594 nm. Experiments as a function of laser power suggest that NV centers and other coexisting defects likely in the form of surface traps contribute to carrier injection, though we find that NVs dominate the system response in the limit of high illumination intensities. Given our growing understanding of near-surface NV centers and adjacent point defects, these results open new perspectives in the application of diamond−liquid interfaces to photocarrier-initiated chemical and spin processes in fluids.
Click for new article in ACS Applied Materials and Interfaces

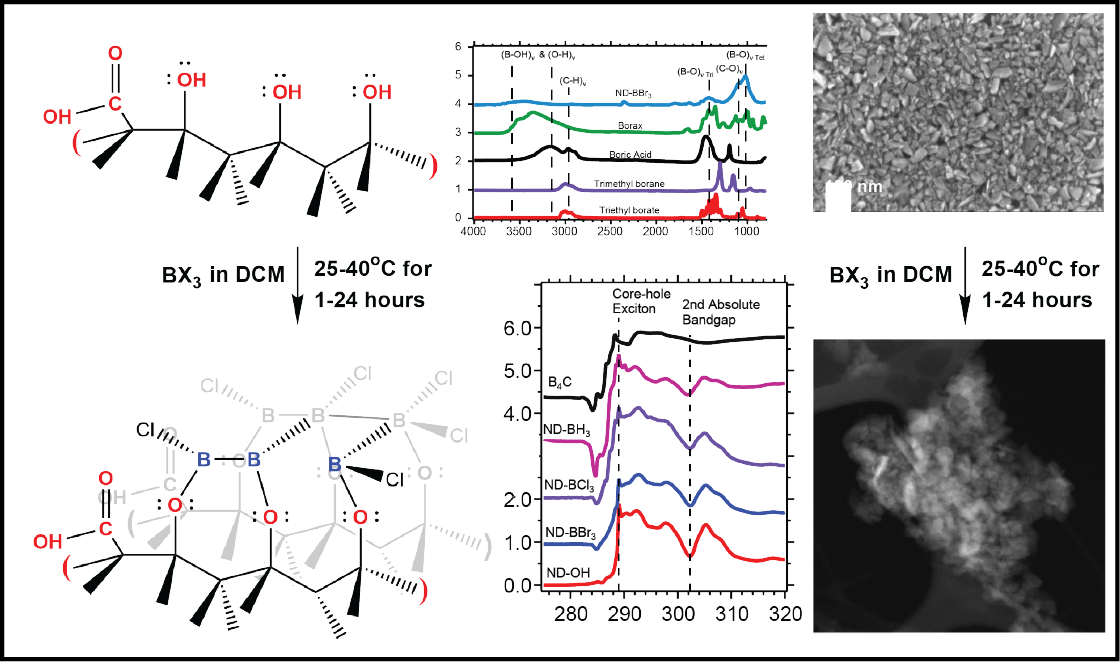
Diamond as a templating substrate is largely unexplored, and the unique properties of diamond including its large bandgap, thermal conductance and lack of cytotoxicity make it versatile in emergent technologies in medicine and quantum sensing. Surface termination of an inert diamond substrate and its chemical reactivity are key in generating new bonds for nucleation and growth of an overlayer material. Oxidized high-pressure high-temperature (HPHT) nanodiamonds (NDs) are largely terminated by alcohols that act as nucleophiles to initiate covalent bond formation when an electrophilic reactant is available. In this work, we demonstrate a templated synthesis of ultrathin boron on ND surfaces using trigonal boron compounds. Boron trichloride (BCl3), boron tribromide (BBr3) and borane (BH3) were found to react with ND substrates at room temperature in inert conditions. BBr3 and BCl3 were highly reactive with the diamond surface, and sheet-like structures were produced and verified with electron microscopy. Surface sensitive spectroscopies were used to probe the molecular and atomic structure of the ND constructs’ surface, and quantification showed the boron shell was less than 1 nm thick after 1–24 hour reactions. Observation of the reaction supports a self-terminating mechanism, similar to atomic layer deposition growth and is likely due to the quenching of alcohols on the diamond surface. The boron-diamond nanostructures were found to aggregate in dichloromethane and were dispersed in various solvents and characterized with dynamic light scattering for future cell imaging or cancer therapy applications using boron neutron capture therapy (BNCT). The unique templating mechanism based on nu-cleophilic alcohols and electrophilic trigonal precursors allows for covalent bond formation and will be of interest to researchers using diamond for quantum sensing, additive manufacturing and BNCT.

SSCL Lab member Anoushka Lakshmi attended a California State University Wide research competition and Placed 1st in the in Physical and Mathematical Sciences – Undergraduate and Graduate Combined category. The presentation was entitled, "Surface Functionalization of HPHT NDs To Increase NVC Photoluminescence Intensity for Targeted Biolabeling." Ms. Lakshmi described nitrogen vacancy center physics and the use of bromine functionalized diamond surfaces as a method to generate new diamond-nitrogen bonds!

Surface chemistry of materials that host quantum bits such as diamond are an important avenue of exploration as quantum computation and quantum sensing platforms mature. Interfacing diamond in general, and nanoscale diamond (ND) in particular with silica is a potential route to integrate the quantum bit into a photonic device, fiber optic, cells or tissues with flexible functionalization chemistry. While silica growth on ND cores has been used successfully for quantum sensing and biolabeling, the surface mechanism to initiate growth was unknown. This report describes the surface chemistry responsible for silica bond formation on diamond and uses X-ray absorption spectroscopy (XAS) to probe the diamond surface chemistry and its electronic structure with increasing silica thickness. A modified Stöber (Cigler) method was used to synthesize 2–35 nm thick shells of SiO2 onto carboxylic acid rich ND cores and the diamond features and surface structure were characterized by overlapping techniques including electron microscopy. Importantly, we discovered that SiO2 growth on carboxylated NDs eliminates the presence of carboxylic acids and that basic ethanolic solutions converts the ND surface to an alcohol-rich surface prior to silica growth. The data supports a mechanism that alcohols on the ND surface generate silyl-ether (ND-O-Si-(OH)3) bonds due to rehydroxylation by ammonium hydroxide in ethanol. Additionally, resonant inelastic X-ray scattering (RIXS) maps produced by the transition edge sensor supports the chemical analysis provided by XAS. Researchers using high-pressure high temperature (HPHT) NDs or any alcohol-terminated material (metal oxides, oxidized silicon carbide or cubic-boron nitride) for quantum sensing applications may exploit these results to design new core-shell quantum sensors with base-catalyzed reactions and metal oxide precursors
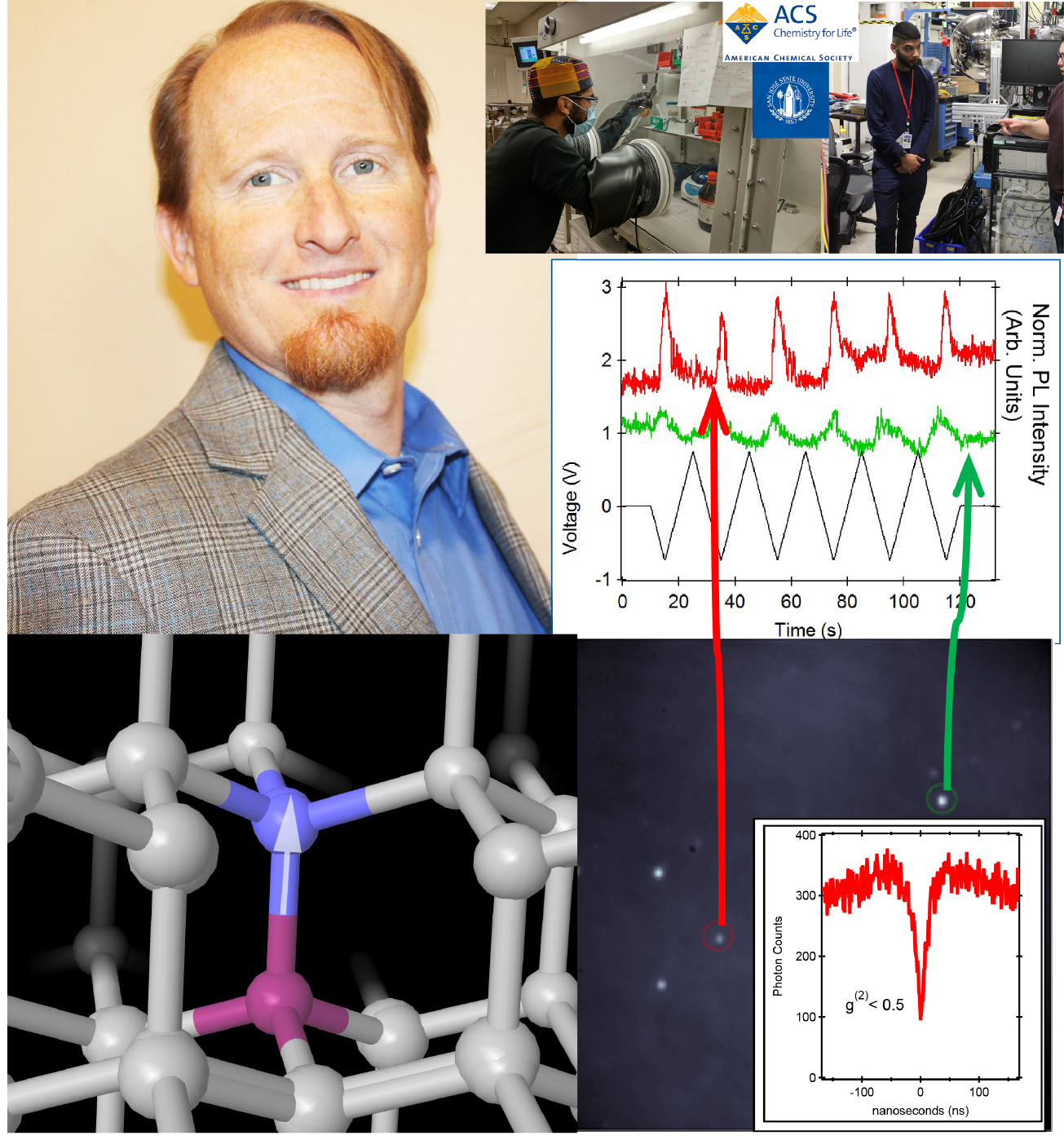
Awarded: Diamond and Nitrogen Vacancy Center Voltage Sensing Funded by NSF
Diamond has many properties that make it valuable well beyond its use as a gemstone. Notably, diamond hosts a sensitive detector of magnetic and electric fields: the nitrogen vacancy (NV) center. Such NV centers can be used to sense magnetic objects and have potential for use as quantum bits (qubits) in the quantum computers of the future. Diamond’s chemical inertness, its reluctance to change or be modified, is both a challenge and an opportunity for materials chemists, physicists and engineers. Changing chemical bonds on the surface of diamond allows for the attachment of molecular groups and anchoring points for measurement and detection. In this LEAPS-MPS project, the PI and his team will explore the surface chemistry of diamond and investigate how the light given off by the NV center changes with an applied voltage. The surface chemistry of nanoscale diamonds and the behavior of the NV center will be simultaneously examined with the long-term aim of making a more advanced quantum sensor. Undergraduate students will learn advanced chemistry and spectroscopy techniques, including use of the Stanford Synchrotron Radiation Lightsource. Recruitment of talent is in collaboration with the Black Leadership and Opportunity Center (BLOC) and other student-based organizations at San Jose State University. This project will provide rigorous and wide-ranging research experiences to prepare scientists and engineers for a successful career by providing the skills to execute a research project, manage troubleshooting, prepare an impactful research presentation and build their resume for future success.

Another great beamline run was realized by Surface Science Center Lab (Wolcott) researchers at the Stanford Synchrotron Radiation Lightsource in collaboration with Staff Scientists Dennis Nordlund and Sang-Jun Lee. Nanodiamond samples were prepared in inert conditions in a glove box and in open air conditions on gold coated silicon wafers and loaded into the ultrahigh vacuum chamber at beamline 10-1. Data was collected on the transition edge sensor and in classic total electron yield mode for Boron, Carbon, Nitrogen, Oxygen, Cobalt, Vanadium and Nickel! SSCL Researchers stayed at the Stanford Guest House and had more than 100 hours of data collection time with 68 samples ranging from nanoscale diamond to negative thermal expansion alloys. Thank you Sang-Jun and Dennis for another fantastic beamline run!! Email Prof. Wolcott about research opportunities (no experience necessary)

Wolcott Lab researcher and Physicist Camron X. Stokes (SJSU Fall 2021) has been awarded an undergraduate award from the American Chemical Society's Division of Colloid and Surface Chemistry!! Camron will receive his award and give an invited talk at the Fall 2022 National Conference in Chicago! Great work Camron !

Bromination of high-pressure high-temperature (HPHT) nanodiamond (ND) surfaces has not been explored and can open new avenues for increased chemical reactivity and diamond lattice covalent bond formation. The large bond dissociation energy of the diamond lattice-oxygen bond is a challenge that prevents new bonds from forming and most researchers simply use oxygen-terminated ND (alcohols and acids) as a reactive species. In this work, we transformed a tertiary alcohol-rich ND surface to an amine surface with 50% surface coverage and was limited by the initial rate of bromination. We observed that alkyl-bromide moieties are highly labile on HPHT NDs and are metastable as previously found using density functional theory. The strong leaving group properties of the alkyl-bromide intermediate were found to form diamond-nitrogen bonds at room temperature and without catalysts. This robust pathway to activate a chemically inert ND surface broadens the modalities for surface termination and the unique surface properties of brominated and aminated ND are impactful to researchers for chemically tuning diamond for quantum sensing or biolabeling applications.
In a collaborative project to advance equity in STEM education, Prof. Abraham Wolcott at SJSU and colleagues from Fresno State Profs. Wei Wu and Vivien Luo,Sonoma State Profs. Sara Kassis and Dean Elizabeth Wade and Prof. Frank Gomez (STEM-NET at Chancellor's Office) received a National Science Foundation award through the division of undergraduate education. The SJSU VR Team includes Jon Oakes of the KLEVR Lab, Nanci Solomon (VR expert) , Patrick Stafford (VR expert developer) and Prof. Wolcott. The goal: Use advanced virtual reality and extended reality technology to advance STEM equity at Cal State and community colleges. The grant will assemble a faculty learning community or FLC to build the use and expertise of educators with extended reality technology. Contact Prof. Wolcott if you are a SJSU faculty member interested in VR for your classroom ($2,500 stipend)! The first cohort will include 3 SJSU, 2 Fresno State, 3 Sonoma and 2 community college faculty....
Prof. Wolcott will be discussing nanoscale diamond surface chemistry, the use of the TES detector and support from the DOD. DOD support has provided for researcher travel expenses, compensation and tuition and has been a vital resource for advancing the careers and experiences of SSCL lab members. Save the date 5/13/2021 and join us! Learn more about the Department of Defense (DoD) programs, CSU awardees, and tips on successfully submitting a DoD proposal. STEM-NET promotes research, community building and innovative educational ideas across the CSU university system and provides students the skills they need to excel in the workforce of the future and meet the needs of California’s innovative and evolving economy.

Apply to the URAP Program at SJSU in the Wolcott Lab for Summer 2021!
Again, The Surface Science Center Laboratory at SJSU (Wolcott Lab) has been selected as the only Cal State University to host Undergraduate Research Apprenticeship Program (URAP) Scholars for Summer 2021! Eligibility: GPA=2.8, STEM Major, US or Naturalized Citizen and ability to commute to SJSU! Summer Stipend is $4500 for 8-10 weeks and NO research experience required. Researchers will perform work on nanoscale diamond with applications in biolabeling, biosensing, magnetometry and quantum computation. African American, Latinx, Native American and Pacific Islanders are encouraged to apply. Apply by March 15th, 2021 Contact Prof. Wolcott for additional info

The Molecular Foundry: DOE operated Nanoscience Center
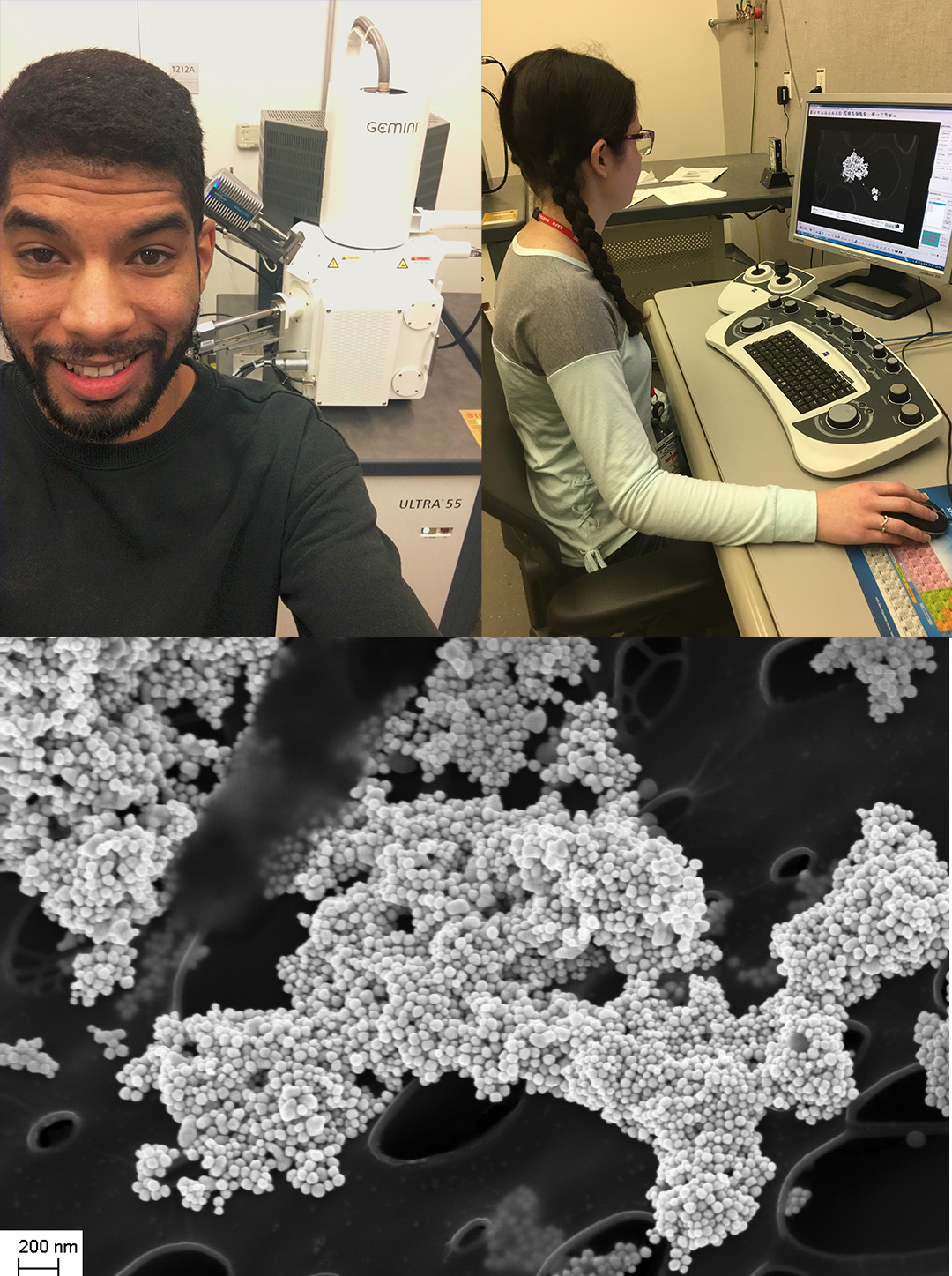
SSCL researchers have access through a user-based proposal system to state-of-the-art instrumentation at The Molecular Foundry and here SSCL reserachers Camron Stokes and Davida Simpson image gold nanoparticle clusters on a Zeiss Ultra-55 in the Imaging and manipulation of nanostructures facility at Lawrence Berkeley National Lab, a Department of Energy Facility.

SSCL SELECTED AS HOST SITE FOR SUMMER 2020 RESEARCH PROGRAM! Deadline March 15th, Apply Today!
The Surface Science Center Laboratory at SJSU (Wolcott Lab) has been selected as the only Cal State University to host Undergraduate Research Apprenticeship Program (URAP) Scholars. Eligibility: GPA=2.8, STEM Major, US or Naturalized Citizen and ability to commute to SJSU! Summer Stipend is $4500 for 8-10 weeks and NO research experience required. Researchers will perform work on nanoscale diamond with applications in biolabeling, biosensing, magnetometry and quantum computation. African American, Latinx, Native American and Pacific Islanders are encouraged to apply. Apply by March 15th! Contact Prof. Wolcott for additional info
X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) is a key technique that the SSCL uses to examine the atomic and molecular structure structure of bulk and nanoscale materials. Here SSCL researchers Grace Jeanpierre, Jocelyn Valenzuela and Cynthia Melendrez collect XPS data.

Interested in Research? Recruitment Seminar for the Wolcott Lab Monday 9/12/2022 in Duncan Hall 5B at 5 pm!Come Learn about the Surface Science Center Lab and the use of materials science with applications in biolabeling, quantum sensing and quantum communication